Explain Why Fluorine and Chlorine Are in the Same Group
The elements that are present in group 17 are fluorine chlorine bromine iodine and astatine. The first electron affinity of oxygen -142 kJ mol-1 is smaller than that of sulphur -200 kJ mol-1 for exactly the same reason that fluorines is smaller than chlorines.

How To Calculate Valency Youtube Gcse Chemistry Chemistry Calculator
Group 17 elements are non-metallic and extensively reactive.

. The nucleus is composed of protons and neutrons. These elements are in the same group in the periodic table due to their electron configurations. The group 17 elements consisting of highly reactive elements are as follows- Fluorine Fl Chlorine Cl Bromine Br Iodine I Astatine As and Tennessine Ts.
Solution for Explain why at room temperature fluorine and chlorine are gases bromine is a liquid and iodine is a solid. 2 c Explain why fluorine and chlorine are in the same group of the periodic table. Group 17 elements called halogens are p block elements present at the second column to the right of the periodic table.
As you might have noticed the first electron affinity of oxygen -142 kJ mol-1 is less. The larger pull from the closer fluorine nucleus is why fluorine is more electronegative than chlorine is. For example iodine is dark voilet in colour and Fluorine is pale yellow in.
Fluorine is the most electronegative element and exhibits only -1 oxidation state. They are called halogens as they react with metals to produce salts. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.
Up to 24 cash back Fluorine í colourless Chlorine í pale green Bromine X orange. It has seven electrons in its outer shell. Group 7A or VIIA of the periodic table are the halogens.
The French scientist André Ampère coined the name fluorine in 1812 Even the great Humphry Davy was unable to produce the element and he became ill by trying to isolate it from hydrofluoric acid. Iodine crystals are shiny purple -. Group 17 occupies the second column from the right in the periodic table and contains fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts.
Halogens are nonmetals with high reactivity. Summarising the trend down the Group. The halogens become darker as you go down the group.
Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. Isotopes are defined as variants of a particular element where these variants will have the same number of protons but differ in the number of neutrons in the atom. A similar reversal of the expected trend happens between oxygen and sulphur in Group 6.
The halogen elements are the six elements in Group 17 of the periodic table. Explain why at room temperature fluorine and chlorine are gases bromine is a liquid and iodine is a solid. Which of the following molecules belongs to the same point group as.
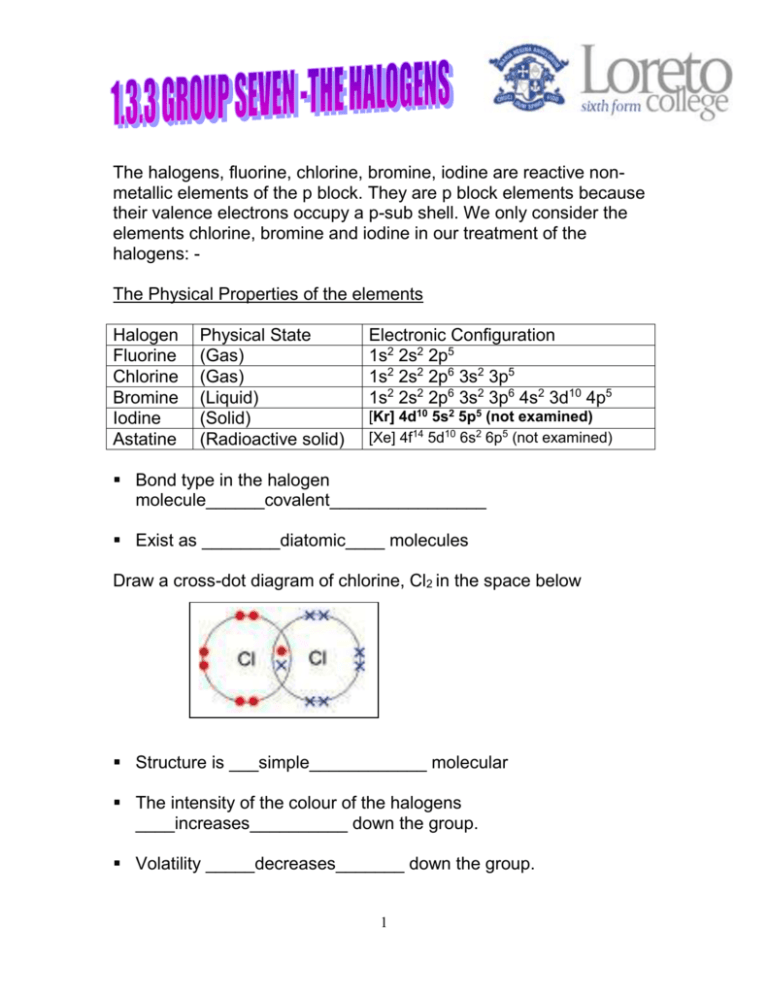
These elements are grouped together as they have similar properties. Fluorine and chlorine are gases bromine is a liquid and iodine is a solid. Since the 2p-subshell is relatively small as compared to 3psubshell the added electron in small 2p-subshell experiences strong interelectronic repulsions in comparison to that in 3p-subshell in Cl.
The early chemists were aware that metal fluorides contained an unidentified element similar to chlorine but they could not isolate it. Group 17 elements fluorine chlorine bromine iodine and astatine are collectively known as. The elements and their compounds have a wide variety of uses.
It gains an electron from another atom in reactions forming a fluoride ion F-. The reason for the atomic mass of chlorine taken as 355u and not as 35u because of the presence of isotopes. Group 17 elements have a variety of colours.
Comparing Group 6 and Group 7 values. Fluorine is in Group 7. Explain why fluorine and chlorine are in the same group of the periodic table.
The most common of these being salt or sodium chloride and the potassium compounds sylvite or potassium chloride and carnallite potassium magnesium chloride hexahydrate. Fluorine F chlorine Cl bromine Br iodine I and astatine At. 3 The elements in Group 17 the halogens show trends in both their chemical and physical properties.
In Fluorine the new electron to be added goes to 2p-subshell while in chlorine the added electron goes to 3p-subshell. The chemical symbol for Fluorine is F. A At room temperature fluorine and chlorine are gases bromine is a liquid and iodine is a solid.
As the halogen atoms get bigger any bonding pair gets further and further away from the halogen nucleus and so is less strongly attracted towards it. The name halogen means salt former derived from the Greek words halo- salt and -gen formation. The Group 7A elements have seven valence electrons in their highest-energy orbitals ns2np5.
Up to 24 cash back so fluorine and chlorine are in the same group because they have the same number of or 7 electrons in their highest energy level or outer shell if no other mark awarded allow 1 mark for have the same similar. Astatine and tennessine are radioactive elements with very short half-lives and thus do not occur naturally. Give the electronic structures of fluorine and chlorine in your explanation.
Chlorine is in group 17 of periodic table also called the halogens and is not found as the element in nature - only as a compound. They both have 7 electrons in their outer shell so therefore are both in group 7 of the periodic table with chlorine directly below fluorine. In other words as you go down the Group the elements become less.
Note that the atom is called fluorine but the. Fluorine is very pale yellow chlorine is yellow-green and bromine is red-brown. Fluorine and chlorine are gases on the other hand bromine is a liquid and iodine is a solid.
I State the trend in the volatility of the Group 17 elements down the group.

Pin By Amurchem On Amazing Chemistry Oxidation State Noble Gas Periodic Tale

What Do We Know About Fluorine Chlorine And Bromine 1 2 3 4 Ppt Download

Alexandra Callaghan Period 7 Science Mr Urso Means That Iodine Bromine Fluorine Chlorine And Astatine Elements Are All Nonmetallic And Strongly Ppt Download
Periodicity Of Group 7 Reactions Activity
Halogens Fluorine Chlorine Bromine Iodine Astatine
1 3 3 Group Vii Booklet Answers

Chlorine Wikipedia The Free Encyclopedia Hydrogen Chloride Metal Organic Framework Electrochemical Cell

Science Coverage Is Cf2cl2 Polar Or Nonpolar Molecular Geometry Covalent Bonding Solubility

State One Reason For Keeping Fluorine And Chlorine In The Same Group Group Of The Periodic Table

David Chalk Teacherchalky1 Twitter Gcse Science Science Daily Science Revision

Explaining Uv Visible Absorption Spectra Of Halogens Spectrum Of Fluorine Chlorine Bromine Iodine Astatine Explaining Different Colours Of Iodine In Different Solventsdoc Brown S Chemistry Revision Notes

Gcse Chemistry Revision Guide 677228862696294620 Gcse Science Gcse Chemistry Revision Chemistry Education

Solved Fluorine Chlorine Bromine And Iodine Are All Halogens Fluorine And Chlorine Are Gases While Bromine Is A Liquid And Iodine Is A Solid At Room Temperature What Type Of Intermolecular Interaction Is

Ionic Bond Definition Examples Formation Chemical Bonding Chemistry Ionic Bonding Chemistry Chemistry 10

Group 7 Halogens Fluorine Chlorine Bromine Iodine Physical Properties Balanced Equations Chemical Reactions Balanced Gcse Chemistry Revision Notes Ks4 Science Igcse O Level

Group 7 Elements The Halogens Group 7 The Halogens The Elements In Group 7 Of The Periodic Table On The Right Are Called The Halogens Fluorine Chlorine Ppt Download

Study Study Study Study Study Side Up 2 00pm Sunday 5th April 2015 Study Notes School Study Tips Study Motivation

Group 7 Elements The Halogens Group 7 The Halogens The Elements In Group 7 Of The Periodic Table On The Right Are Called The Halogens Fluorine Chlorine Ppt Download

Comments
Post a Comment